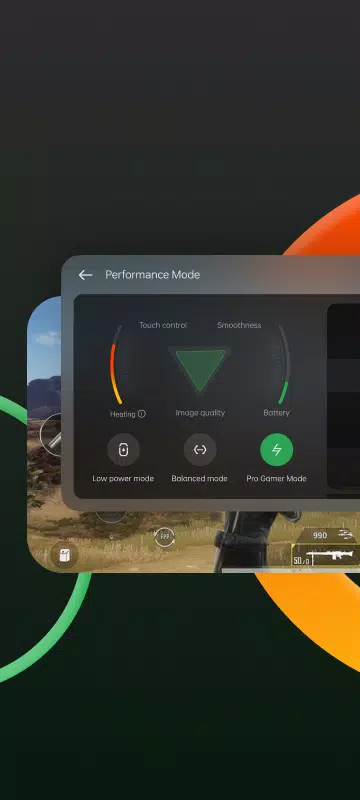
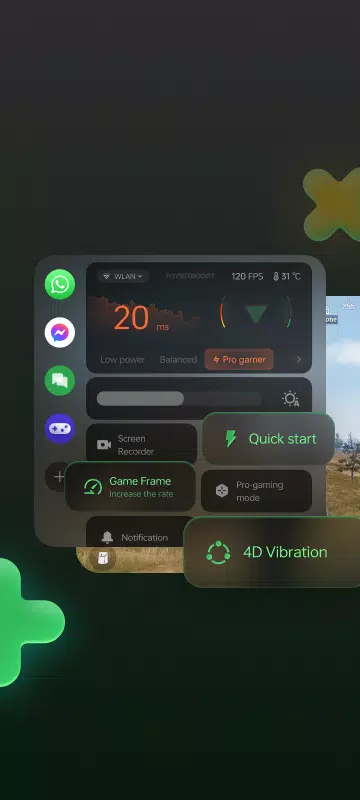
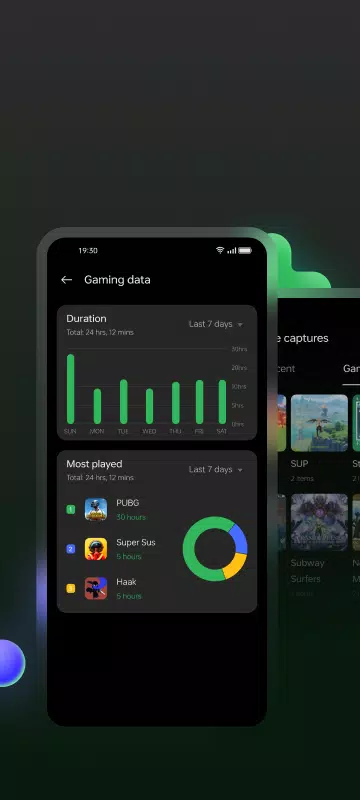
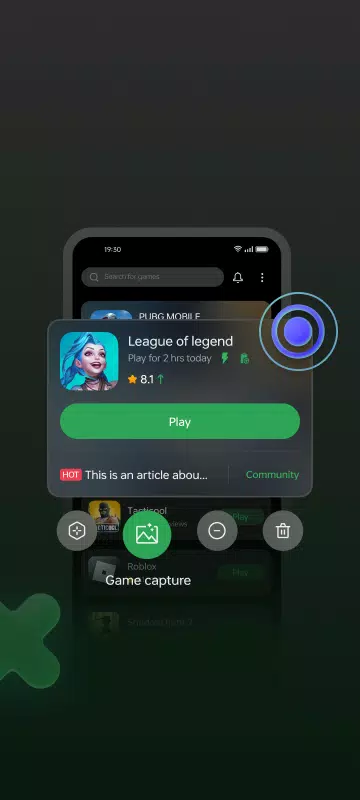
Lar > Aplicativos > Entretenimento > Game Space

| Nome do aplicativo | Game Space |
| Desenvolvedor | ColorOS |
| Categoria | Entretenimento |
| Tamanho | 77.0 MB |
| Última versão | 5.11.0_space |
| Disponível em |
What is a Casino? How to Choose the Best One for You
A casino is a fun place to play games and try to win money. However, there are some things you should know before you head to the nearest one. These include what to expect, how to choose the best one for you, and other tips. You can also find out what games are played at a casino, and what the House edge is. This article will help you choose the best place to play for you. Hopefully, you’ll have a great time!
Common casino games
Slot machines are among the most popular casino games. They are very easy to play, but they can also be very frustrating, as the odds are stacked against you. The probability of winning is one in three, so playing slots is not a good idea unless you want to lose money. Despite this, slot machines are the most popular games at most casinos. However, beware of their high house edges. Here are some tips to help you win more often at the slot machines.
Some casinos offer other forms of sports betting, such as keno. This game is similar to bingo, but involves drawing numbers and trying to match them to the numbers on the card. Many casinos also have live keno rooms, and some even offer virtual keno games. These games are not as widely available, but they’re still popular. If you’re unsure about the rules of keno, be sure to ask a casino employee.
Cost of gambling at a casino
The average cost of problem gambling at a casino is around $1,000 per person per year. However, this figure does not include the costs of lost work days, lost productivity, or crime. Additionally, problem gambling also affects people who are not themselves affected by gambling. In fact, one problem gambler can affect five to ten other people. In other words, a person can lose as much as two percent of his or her income from gambling at a casino.
The social costs associated with gambling can be measured indirectly, through the costs of mental illness and crime. For example, crime related to gambling may result in higher police costs, and the costs of court cases and probation are often passed on to taxpayers. However, measuring such costs is difficult, and the research that has been done has used various methods. Some studies rely What is the most likely product of the following reaction?
- A: 3-amino-1-propanol
- B: 1,3-propanediamine
- C: 3-amino-1-cyclohexanol
- D: 1,3-cyclohexanediamine
The given reactant is 1,3-cyclohexanedicarboxylic acid.
The compound reacts with hydrazoic acid $\left( \text{HN}{3} \right)$ in the presence of conc. $\text{H}{2}\text{SO}_{4}$, which corresponds to the Schmidt reaction.
The Schmidt reaction involves the reaction of carboxylic acids with hydrazoic acid, leading to the formation of amines with the loss of a carbon atom. Specifically, a monocarboxylic acid yields an amine with one less carbon: $\text{R-COOH} + \text{HN}{3} \xrightarrow[\text{H}{2}\text{SO}{4}]{\text{conc.}} \text{R-NH}{2} + \text{CO}{2} + \text{N}{2}. For a dicarboxylic acid, the reaction can lead to a diamine with loss of two carbon atoms.
Given that the reactant is a dicarboxylic acid (1,3-cyclohexanedicarboxylic acid, i.e., cyclohexane with two -COOH groups at positions 1 and 3), the Schmidt reaction would convert both -COOH groups into -$\text{NH}_{2}$ groups, with the loss of two carbon atoms.
Let's consider the structure: Cyclohexane ring with two carboxylic acid groups at positions 1 and 3. After Schmidt reaction, each -COOH becomes -$\text{NH}{2}$, so the product should be a cyclohexane ring with two amino groups at positions 1 and 3. But careful: In Schmidt reaction, the carbon of the carboxylic acid group is lost (as $\text{CO}{2}$), so the amine nitrogen attaches directly to the carbon that was originally attached to the carboxylic acid group. However, for a dicarboxylic acid, if both -COOH groups undergo Schmidt reaction, both carbons of the carboxyl groups are lost. This means that the carbon atoms that were bonded to the -COOH groups now become bonded to -$\text{NH}_{2}$ groups directly. So the product is a cycloalkane with two amino groups on the ring, but the ring size remains the same? Actually, careful: When a carboxylic acid undergoes the Schmidt reaction, the carbonyl carbon is replaced by nitrogen. The product is R-NH2, where R is the rest of the molecule. So if we have a dicarboxylic acid, let's denote it as HOOC-R-COOH, after Schmidt reaction, it becomes H2N-R-NH2, with the two carboxyl carbons removed. In our case, the starting compound is 1,3-cyclohexanedicarboxylic acid. That means that the carboxylic acid groups are attached to the cyclohexane ring at positions 1 and 3. So after Schmidt reaction, the carboxyl groups are converted into amino groups, so we get 1,3-cyclohexanediamine. That is the product. However, note that in the Schmidt reaction, the carbon of the carboxyl group is lost. So the amino group is attached to the same carbon to which the carboxyl was attached. So the structure is cyclohexane with -NH2 at positions 1 and 3. So it remains a 6-membered ring with two amino groups. So the product should be 1,3-cyclohexanediamine.
Now looking at the options: A: 3-amino-1-propanol (that is an acyclic compound with 3 carbons, amino at one end, hydroxyl at the other) B: 1,3-propanediamine (acyclic 3-carbon diamine) C: 3-amino-1-cyclohexanol (cyclohexane with amino and hydroxyl groups at 1 and 3) D: 1,3-cyclohexanediamine
Thus, D is the correct product.
But we should check: Is there any possibility that the ring undergoes contraction? Actually, the Schmidt reaction of carboxylic acids does not involve ring contraction unless it is a cyclic ketone or something. For carboxylic acids, it's a simple substitution of COOH by NH2 with loss of CO2. However, if the carboxylic acid is part of a ring, like in cyclohexanecarboxylic acid, the product is cyclohexylamine (the ring remains six-membered). So in the case of a dicarboxylic acid on a ring, both carboxyls get converted to amines, so we get a diamine on the same ring. So the product is 1,3-cyclohexanediamine.
Thus, the answer is D: 1,3-cyclohexanediamine.
But wait: There is an option C: 3-amino-1-cyclohexanol, which is not a diamine but an amino alcohol. That might arise if only one carboxyl group reacts, but in the given conditions both would likely react. Also, the Schmidt reaction with dicarboxylic acids can sometimes give different products like diamines or cyclic ureas, but typically with conc. H2SO4, it yields the diamine. So D is correct.
Thus, the most likely product is 1,3-cyclohexanediamine.
So the correct answer is D.
-
 Marvel Rivals Data de lançamento da primeira temporada revelada
Marvel Rivals Data de lançamento da primeira temporada revelada
-
 Sonic Racing: Crossworlds Personagens e faixas reveladas para o próximo teste de rede fechada
Sonic Racing: Crossworlds Personagens e faixas reveladas para o próximo teste de rede fechada
-
 <🎜 🎜 🎜 🎜 🎜 🎜>
<🎜 🎜 🎜 🎜 🎜 🎜>
-
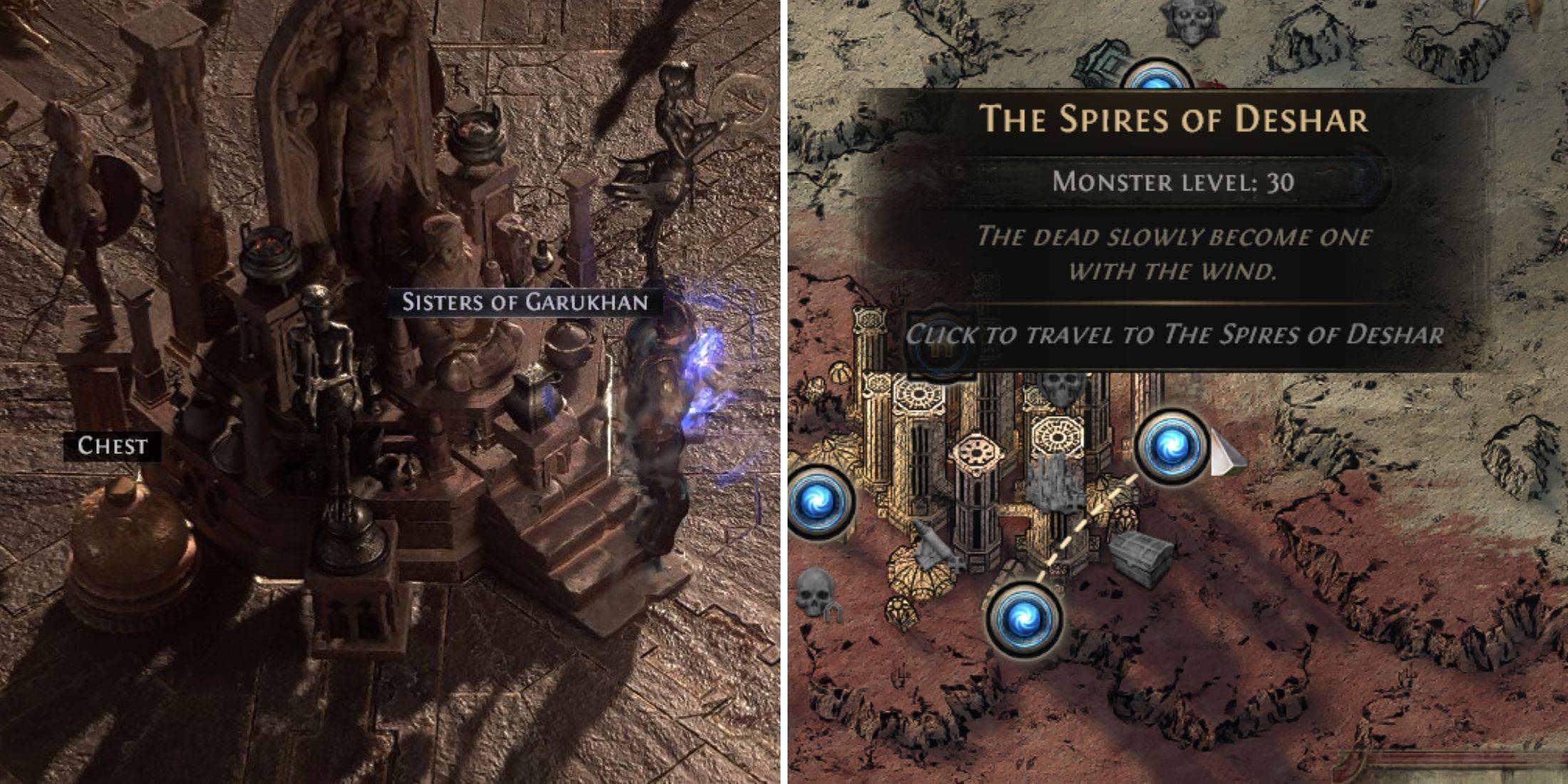 Anunciando Caminho do Exílio 2: Guia para a expansão das irmãs de Garukhan
Anunciando Caminho do Exílio 2: Guia para a expansão das irmãs de Garukhan
-
 Ubisoft cancela acesso antecipado a Assassin's Creed Shadows
Ubisoft cancela acesso antecipado a Assassin's Creed Shadows
-
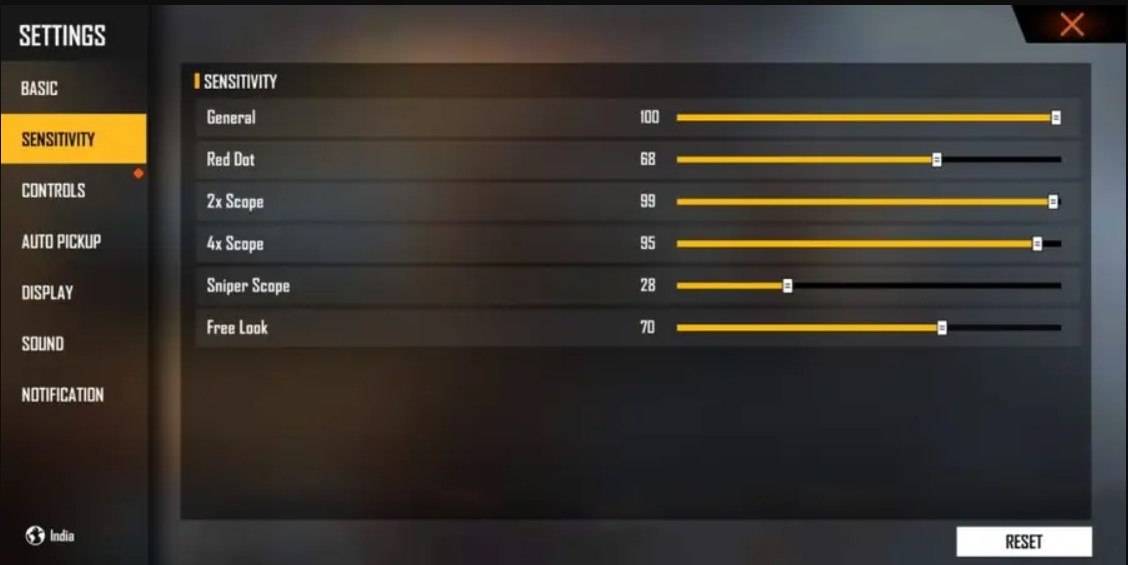 Configurações ótimas de incêndio livre para domínio da cabeça
Configurações ótimas de incêndio livre para domínio da cabeça